Introduction:
CAR T-cell therapy has made a significant impact in the treatment of relapsed and refractory (R/R) DLBCL. However, there are significant barriers to timely administration of CAR T. Vein-to-vein time (time between leukapheresis and CAR T infusion) is an important barrier reported by clinical trials. In the real world, administration of CAR T may be further delayed due to financial clearance or single case agreement (SCA), shortage of leukapheresis slots, disease progression and death, among others. The time between CAR T referral and infusion (brain-to-vein) in the real-world is unknown. Understanding the barriers to timely administration of CAR T is crucial to expanding the access to CAR T for patients with R/R DLBCL.
Methods:
Adult patients (pts) with R/R DLBCL who underwent consultation for CAR T at our center from 2018 to 2022 were included in the study. The following information was collected from each subject and analyzed with descriptive statistics: referral type (internal/external), insurance type (private/government), private insurance subtype (SCA/non-SCA), and CAR T product (liso-cel/axi-cel/clinical trial/non-conforming product). For continuous variables, we reported the median and range, while categorical variables were reported as frequencies and percentages. The brain-to-vein time was classified as high (70-130 d), medium (45-70 d), and low (27-45 d). The effects of variables on brain-to-vein time were analyzed by Fisher's exact test for categorical variables, and Wilcoxon rank-sum test for continuous variables. Survival analyses were performed using the Kaplan-Meier and Cox Proportional Hazard model.
Results:
The study included 78 consecutive pts who were referred for consideration of CAR T for DLBCL. Thirteen (16.6%) pts did not receive CAR T therapy due to disease progression and death (n=5), lack of social support or caregiver (n=4), lack of financial clearance (n=2), and complete response to bridging therapy (n=2).
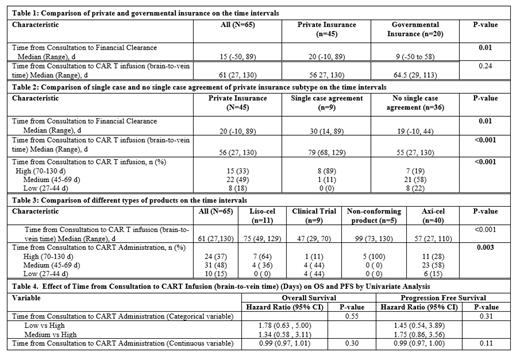
Sixty-five (83.3%) pts received CAR T therapy. Median age was 61 y (27-83), 72% were male, and 18.4% belonged to racial or ethnic minority groups. The median time (range) from consultation to financial clearance, leukapheresis, and CAR T infusion were 15 (-50, 89), 30 (3, 91) and 61 d (27, 130), respectively. For some pts financial clearance was obtained prior to consultation. The median vein-to-vein time was 31 d (16, 76). There was no difference in brain-to-vein time (p=0.66) for external and internal referrals. Although pts with private insurance took longer to obtain financial clearance compared to pts with government insurance (median, 20 vs 9 d, p=0.01) (Table 1), there was no significant difference in the brain-to-vein time between the 2 groups (p=0.24). 20% of pts with private insurance needed SCA. Compared to those who did not need SCA, pts who needed SCA had a significant delay in time from consultation to financial clearance (median, 30 vs 19 d, p=0.01) and CAR T infusion (median 79 vs 55 d, p<0.001) (Table 2). Brain-to-vein time was significantly different among different products, with clinical trial the shortest (47 d, n= 9), non-conforming products the longest (99 d, n=5), and axi-cel (57 d, n=40), liso-cel (75 d, n=11) in between (p<0.001; Table 3). Of the 5 pts who received non-conforming products, 4 were intended for liso-cel and 1 for axi-cel. Brain-to-vein time did not significantly impact the progression-free survival [hazard ratio (HR)=0.988, 95% confidence interval (CI) = 0.973-1.003, p=0.11) or overall survival (HR=0.990, 95%CI=0.972-1.009, p=0.30) (Table 4). There was concordance when the time from consultation to CAR T therapy was used as a continuous variable and when it was separated into low, medium, and high quartiles and tested as categorical variables (Tables 2-4).
Conclusion:
We have identified multiple factors that prolonged brain-to-vein time for CAR T-cell therapy in the real-world. Pts whose insurance required SCA or who received non-conforming products had significant delay in receiving CAR T. Although delay in receiving CAR T therapy did not impact survival at our institution, several patients who could have benefited from CAR T did not receive it due to disease progression/death (6.4%) or lack of financial clearance (2.6%). Rapid financial clearance and eliminating the need for SCA could abbreviate the brain-to-vein time and expand access to patients with rapidly progressive DLBCL.
Disclosures
Moyo:Kite Pharmaceuticals: Consultancy. Jacobs:Beigene: Consultancy, Speakers Bureau; Pharmacyclics: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Teneobio: Research Funding; Adaptive: Consultancy; Genentech: Consultancy; SecuraBio: Consultancy, Speakers Bureau; AbbVie: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Research Funding, Speakers Bureau; LOXO Oncology: Research Funding. Park:Seattle Genetics: Research Funding; BMS: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees; Epizyme: Membership on an entity's Board of Directors or advisory committees. Ghosh:AstraZenca, Janssen, Pharmacyclics, Kite pharma, BMS, Epizyme: Speakers Bureau; see consulting and speaker's bureau.: Honoraria, Other: see consulting and speaker's bureau. ; TG Therapeutics, Genentech/Roche, Bristol Myers Squibb,Gilead, Morphosys, AbbVie, Pharmacyclics,: Research Funding; Roche NHL soultions panel: Membership on an entity's Board of Directors or advisory committees; Seagen, TG Therapeutics, AstraZeneca, Phamacyclics, Janssen, Bristol Myers Squibb, Gilead Sciences, Kite Pharma, Beigene, Incyte, Lava Therapeutics, Incyte, Roche/Genentech Novartis, Loxo Oncology, AbbVie, enmab, Adaptive Biotech, ADC Therapeutics: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal